HeberNasvac®, an option in the treatment of chronic hepatitis B
Abstract
Introduction: Cuba produces the HeberNasvac® vaccine for the treatment of chronic hepatitis B. Its relevance lies in achieving sustained virological control results in a greater proportion of patients.
Objective: To evaluate the safety and effectiveness of the therapeutic vaccine HeberNasvac® in the treatment of chronic hepatitis B, in the province of Camagüey in the period from January 2019 to December 2020.
Methods: A quasi-experimental study of therapeutic intervention was carried out in patients treated at the Provincial Consultation of Chronic Viral Hepatitis. The universe consisted of 24 adult patients, with detectable viral load at the beginning of the study. The primary source of the investigation was given by the medical history.
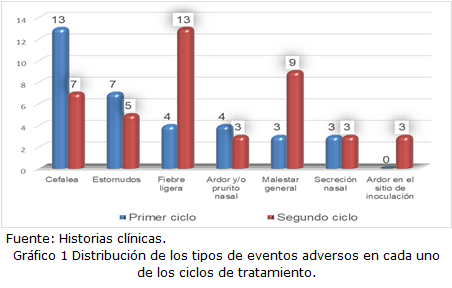
Results: In the first cycle, headache and sneezing were more prevalent; and in the second cycle, fever and general malaise were more prevalent. At the end of the treatment, the majority showed improvement in the results of liver function tests. Before treatment, the largest number of patients had a detectable viral load above 250 copies / mL and after receiving treatment, several of the cases resulted in an undetectable load. The treatment was considered to be of medium safety in the largest number of patients and the effectiveness was high.
Conclusions: There were more adverse events in the second cycle of treatment. Liver function tests showed improvement at the end of treatment. The viral load after treatment showed a decrease. The treatment showed medium safety and high effectiveness.
DeCS: HEPATITIS B, CHRONIC/drug therapy; DRUG-RELATED SIDE EFFECTS AND ADVERSE REACTIONS; HEPATITIS B VACCINES/therapeutic use; SEXUALLY TRANSMITTED DISEASES; SIGNS AND SYMPTOMS.
Downloads
References
1. Organización Panamericana de la Salud [Internet]. Washington, D.C: OPS; ©2020 [citado 13 Oct 2021]. Día Mundial de la Hepatitis 2020. Un Futuro sin Hepatitis. Disponible en: https://www.paho.org/es/noticias/28-7-2020-dia-mundial-hepatitis-2020-futuro-sin-hepatitis
2. Ministerio de Salud Pública. Anuario Estadístico de Salud 2020 [Internet]. La Habana: Dirección de Registros Médicos y Estadísticas de Salud;2021 [citado 13 Oct 2021]. Incidencia de hepatitis viral aguda según tipo y provincia.2020;[aprox. 1 p.]. Disponible en: https://files.sld.cu/bvscuba/files/2021/08/Anuario-Estadistico-Espa%C3%B1ol-2020-Definitivo.pdf
3. Otero W, Parga J, Gastelbondo J. Serología del virus de la hepatitis B: para múltiples escenarios, múltiples exámenes. Rev Col Gastroenterol [Internet]. 2018 Oct-Dic [citado 12 May 2021];33(4):411-22. Disponible en: https://www.redalyc.org/articulo.oa?id=337758126009
4. Ramírez F, Miranda J, Venegas M. ComparisonbetweenXpert® and COBAS® TaqMan® forthemeasuringof viral load of hepatitis B and C viruses. Rev chil infectol [Internet]. 2021 Jun [citado 25 Sep 2021];38(3):344-48. Disponible en: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0716-10182021000300344&lng=es
5. Ministerio de Salud Pública. Plan Estratégico Nacional para la prevención y control de las ITS, el VIH las hepatitis 2019-2023. Resolución Ministerial No. 56 [Internet]. La Habana:MINSAP;2019 [citado 01 Oct 2020]. Disponible en: http://legislacion.sld.cu/index.php?P=DownloadFile&Id=682
6. Gara N, Abdalla A, Rivera E, Zhao X, Werner JM, Liang TJ, et al. Durability of antibody response following hepatitis B vaccination in healthcare workers vaccinated as adults. Hepatology [Internet]. 2012 [citado 27 May 2021];56(1):905. Disponible en: https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/hep.26040
7. Sánchez González C. HeberNasvac®(vacuna recombinante terapéutica contra la infección por el virus de la hepatitis B). Rev Cubana Farm [Internet]. 2020 [citado 25 Sep 2021];53(1):[aprox. 9 p.]. Disponible en: http://www.revfarmacia.sld.cu/index.php/far/article/view/425
8. Aguilar Rubido JC, Boctor KH. The 26th Conference of the Asian Pacific Association for the Study of the Liver (APASL 2017). Biotecnol Apl [Internet]. 2017 Ene-Mar [citado 25 Sep 2021];34(1). Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1027-28522017000100007
9. Aguilar Rubido J, Guillén Nieto G, Pentón Arias E, Fazle Akbar S, Al-Mahtab M, Michel ML, et al. Impacto de la ruta de inmunización intranasal en el mecanismo de acción farmacológica de la vacuna terapéutica HeberNasvac. Resultados en un modelo murino con el virus de la hepatitis B y en pacientes con hepatitis B crónica. Anales de la Academia de Ciencias de Cuba [Internet]. 2019 [citado 25 Sep 2021];9(3). Disponible en: http://www.revistaccuba.cu/index.php/revacc/article/view/725
10. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos. Resumen de las Características del Producto. HeberNasvac® [Internet]. La Habana: CECMED; 2019 [citado 12 May 2021]. Disponible en: https://www.cecmed.cu/sites/default/files/adjuntos/rcp/biologicos/rcp_hebernasvac_0.pdf
11. Qi X, An M, Wu T, Jiang D, Peng M, Wang W, et al. Transient elastography for significant liver fibrosis and cirrhosis in chronic hepatitis B: A meta-analysis. Can J Gastroenterol Hepatol [Internet]. 2018 [citado 25 Sep 2021];3406789:1-13.Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994263/pdf/CJGH2018-3406789.pdf
12. Lian MJ, Zhang JQ, Chen SD, Zhang DD, Yang YY, Hong GL. Diagnosticaccuracyofγ-glutamyltranspeptidase-to-platelet ratio forpredicting hepatitis B-related fibrosis: a meta-analysis. Eur J GastroenterolHepatol. [Internet]. 2019 May [citado 25 Sep 2021];31(5):599-606. Disponible en: https://pubmed.ncbi.nlm.nih.gov/30807447/
13. Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int [Internet]. 2019 Oct [citado25 Sep 2021];39(10):1868-75. Disponible en: https://pubmed.ncbi.nlm.nih.gov/31136052/
14. Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol [Internet]. 2018 Abr [citado 25 Sep 2021];68(4):672-81. Disponible en: https://pubmed.ncbi.nlm.nih.gov/29756595/
15. Terrault N, Lok AS, McMahon B, Chang K, Hwang J, Jonas M, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology [Internet]. 2018 Abr [citado 25 Sep 2021];67(4):1560-99. Disponible en: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.29800
16. Rodríguez Butib M, Esteban R, Lens Sabela, Prieto M, Suárez E, García-Samaniego J. Documento de consenso de la Asociación Española para el Estudio del Hígado sobre el tratamiento de la infección por el virus de la hepatitisB. Gastroenterología y Hepatología [Internet]. 2020 [citado 25 Sep 2021];49(9):559-87. Disponible en: https://www.elsevier.es/es-revista-gastroenterologia-hepatologia-14-pdf-S0210570520301588
17. Crespo J, Cuadrado A, Perelló C, Cabezas J, Llerena S, Llorca J, et al. Epidemiology of hepatitis C virus infection in a country with universal access to direct-acting antiviral agents: Data for designing a cost-effective elimination policy in Spain. J Viral Hepat [Internet]. 2019 [citado 25 Sep 2021];3(8):6-11. Disponible en: https://pubmed.ncbi.nlm.nih.gov/31755634/
18. Bonacci M, Lens S, Marino Z, Londoño MC, Rodríguez Tajes S, Mas A, et al. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment Pharmacol Ther [Internet]. 2018 May [citado 25 Sep 2021];47(10):1397-1408. Disponible en: https://pubmed.ncbi.nlm.nih.gov/29577350/
19. Gordillo Hernández A. Marcadores serológicos de infección por el virus de la hepatitis B en estudiantes de la Escuela Latinoamericana de Medicina. Arch méd Camagüey [Internet]. 2018 [citado 29 Sep 2021];22(5):694-707. Disponible en: http://scielo.sld.cu/pdf/amc/v22n5/1025-0255-amc-22-05-694.pdf
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Yosvany Rojas-Peláez, Zaimara San-José-Maceo, Mayelin Hernández-Rodríguez, Emilia Argelia Don-Quirós, Ernesto Smith-López, Yon Luis Trujillo-Pérez

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright: Camagüey Medical Archive Magazine, offers immediately after being indexed in the SciELO Project; Open access to the full text of the articles under the principle of making available and free the research to promote the exchange of global knowledge and contribute to a greater extension, publication, evaluation and extensive use of the articles that can be used without purpose As long as reference is made to the primary source.
Conflicts of interest: authors must declare in a mandatory manner the presence or not of conflicts of interest in relation to the investigation presented.
(Download Statement of potential conflicts of interest)
The Revista Archivo Médico de Camagüey is under a License Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 International (CC BY 4.0).
This license allows others to distribute, to mix, to adjust and to build from its work, even for commercial purposes, as long as it is recognized the authorship of the original creation. This is the most helpful license offered. Recommended for maximum dissemination and use of licensed materials. The full license can be found at: https://creativecommons.org/licenses/












 22 julio 2025
22 julio 2025