Caracterization of patiens with basal cell carcinoma treated with HeberFERON
Abstract
Background: basal cell carcinoma is the most frequent skin cancer. The treatment of choice is surgical, but there are other therapies. HeberFERON is a pharmaceutical formulation containing a mixture of interpheron alpha2b and IFN-Y in synergistic proportions of anti-tumor activity.
Objective: to characterize patients with basal cell carcinoma treated with HeberFeron.
Methods: a transversal, observational, descriptive study was carried out in which 22 patients were clinically and histologically diagnosed with basal cell carcinoma, who attended a Dermatology consultation at the University Hospital "Manuel Ascunce Domenech", Camagüey, Cuba. 3.5 million IU of HeberFeron, was administered, near the lesion, three times a week on alternate days for three weeks, followed biweekly for 13 weeks, with final evaluation at week 16. The variables studied were: sex, skin photo-type, tumor site, size of lesions, clinical subtype, occupation, clinical response, cosmetic effect and adverse reactions. The information obtained was processed using the statistical package SPSS v21. The methods used were descriptive statistics with distribution of absolute and relative frequencies. The results were presented in tables and graphs.
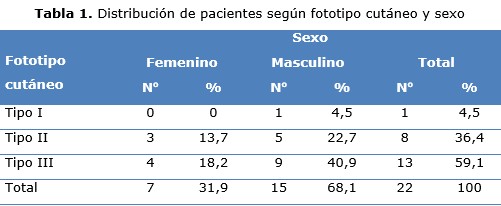
Results: male sex, cutaneous photo-type III, predominated in more than half of the patients. Face lesions predominated in more than four fifths of them, and almost two thirds measured less than two centimeters. The nodular clinical subtype prevailed in half of these, just like workers exposed to the sun. All had a favorable clinical response, with a complete response in two thirds, and partial in a third, as well as an acceptable cosmetic effect. Most presented chills as an adverse reaction, followed by fever.
Conclusions: the HeberFERON was an effective and safe medicine to treat basal cell carcinoma, and offer an alternative in patients who cannot be operated on.
DeCS: INTERFERON ALPHA-2/therapeutic use; CARCINOMA, BASAL CELL /surgery; CARCINOMA, BASAL CELL/drug therapy; NEOPLASMS, BASAL CELL/drug therapy; IMMUNOMODULATION/drug effects.
Downloads
References
1. Chanussot Depress C, Arenas R, Vega Memije M. Cánceres de piel más frecuentes en el Hospital General de Pemex de Veracruz. Dermatología CMQ. 2014;12(1):13–17.
2. Gómez Cisneros P, Tschen J. Carcinoma de células escamosas limitado a la tinta roja de un tatuaje. Dermatología CMQ. 2015; 13(1):37–39.
3. Alcalá Pérez D, Carmona Contreras FP, González Gutiérrez JF. Carcinoma basocelular agresivo. Dermatología CMQ. 2018; 16(2):134-137.
4. Bernia E, Llombart B, Serra-Guillén B, Bancalari E, Nagore E, Requena C, et al. Experiencia con vismodegib en carcinoma basocelular avanzado en un centro oncológico. Actas dermosifiliogr.2018;109(9):813-820.
5. Florez Morales I, Bertel Rodríguez D, Correa Londoño LA, Velásquez López MM. Reporte de la experiencia de la Sección de Dermatología de la Universidad de Antioquia en la quinta jornada de detección de cáncer de piel, Asocolderma 2016. Iatreia [Internet]. 2018 [citado 14 Oct 2019];31(4):[aprox. 8 p.]. Disponible en: http://www.scielo.org.co/pdf/iat/v31n4/0121-0793-iat-31-04-00362.pdf
6. McCalmont TH. The shape of basal cell carcinoma. J Cutan Pathol.2014;41(3):283-5.
7. Lohuis PJ, Joshi A, Borggreven PA, Vermeeren L, ZupanKajcovski B, Al-Mamgani A, et al. Aggresive basal cell carcinoma of the head and neck, challenges in surgical management. Eur Arch Otorhinolaryngol. 2016;273(11):3881-89.
8. Vega Gonzales LG. Carcinoma basocelular. Tratamiento con interferonalfa2b intralesional. Dermatología CMQ. 2016;14(2):100-105.
9. Griffin LL, Ali FR, Lear JT. No melanoma skin cancer. Clin Med (Lond) [Internet]. 2016 [citado 14 Oct 2017];16(1):[aprox. 8 p.]. Disponible en: http://www.clinmed.rcpjournal.org/content16/1/62.long
10. Bello Rivero I, García Vega Y, Valenzuela Silva C, Bello Álvarez C, Vázquez Blomquiat D, López Saura P. Development of a new formulation of interferons (HBERPAG) for BCC treatment. J Res Ther. 2013;1(10):235-243.
11. García Vega Y, Anasagasti Angulo L, Valenzuela Silva C, Navarro Mestre M, Maribeth Ordoñez S, Acosta Medina D, et al. Retrospective study of periocular non melanoma skin cáncer treated with the combination of INF alpha2b and Gamma (HeberPAG). J Clinic Exp Ophthalmol. 2015;6(5):1-8.
12. Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: resived RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2): 228-47.
13. Alonso Canul ME, Eljure López N, Calderón Rocher C, Rubio Zapata H, Proy Trujillo H, Plasencia Gómez A, et al. Cáncer de piel en Yucatán: un estudio epidemiológico de 10 años. Dermatología CMQ. 2015;13(1):7–39.
14. Adachi K, Yoshida Y, Noma H, Goto H, Yamamoto O.Characteristics of multiple basal cell carcinomas: The first study on Japanese patients. J Dermatol. 2018;45(10):1187-1190.
15. Castañeda Gamerosa P, Juliana Eljure Téllez J. El cáncer de piel, un problema actual. Rev Fac Med UNAM [Internet]. 2016 [citado 30 Nov 2019];59(2):[aprox. 9 p.]. Disponible en: http://www.medigraphic.com/pdfs/facmed/un-2016/un162b.pdf
16. Garza Chapa JI, Vázquez Martínez O, Garza Rodríguez V, Vázquez Herrera NE, Espinoza González N, Ocampo Candiani J. Neoformaciónexofítica en el hombro derecho. Dermatol Rev Mex [Internet]. 2016 Mar [citado 30 Nov 2019];60(2):[aprox. 4 p.]. Disponible en: http://www.medigraphic.com/pdfs/derrevmex/rmd-2016/rmd162k.pdf
17. Jiménez Barbán Y, Vega Pupo C, Vila Pinillo D, Fernández Ychaso G, Arias Núñez V, Bello Rivero I. Uso de HeberPAG en carcinoma basocelularperiocular. Rev Cubana Oftalmol [Internet]. 2014 Sep [citado 30 Nov 2019];27(3):[aprox. 8 p.]. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-21762014000300014&lng=es
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Telma Margarita Ferrá-Torres, Edward Stive Sánchez-Rodríguez, Yoddali Ballester-Caballero, Karen Sallary-Gutiérrez

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright: Camagüey Medical Archive Magazine, offers immediately after being indexed in the SciELO Project; Open access to the full text of the articles under the principle of making available and free the research to promote the exchange of global knowledge and contribute to a greater extension, publication, evaluation and extensive use of the articles that can be used without purpose As long as reference is made to the primary source.
Conflicts of interest: authors must declare in a mandatory manner the presence or not of conflicts of interest in relation to the investigation presented.
(Download Statement of potential conflicts of interest)
The Revista Archivo Médico de Camagüey is under a License Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 International (CC BY 4.0).
This license allows others to distribute, to mix, to adjust and to build from its work, even for commercial purposes, as long as it is recognized the authorship of the original creation. This is the most helpful license offered. Recommended for maximum dissemination and use of licensed materials. The full license can be found at: https://creativecommons.org/licenses/












 22 julio 2025
22 julio 2025