Marcadores serológicos de infección por el virus de la hepatitis B en estudiantes de la Escuela Latinoamericana de Medicina
Palabras clave:
Palabras clave, Marcadores serológicos VHB, serología de VHB, prevalencia de VHB, virus de la hepatitis B.Resumen
Fundamento: la hepatitis B es un importante problema de salud a nivel mundial. Según la OMS, se estima que 2 000 millones de personas han sido infectadas y más de 360 millones son portadores crónicos. El diagnóstico de la hepatitis B se ha realizado a través de técnicas serológicas para detectar diferentes marcadores virales.
Objetivo: determinar el comportamiento de los marcadores serológicos del virus de la hepatitis B (VHB) en estudiantes de la Escuela Latinoamericana de Medicina durante el primer trimestre de 2017.
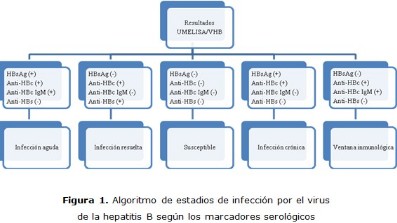
Métodos: se realizó un estudio descriptivo de corte transversal entre enero y marzo de 2017, con estudiantes de la Escuela Latinoamericana de Medicina. Se estudiaron 762 muestras clínicas que se recibieron en el Centro Provincial de Higiene, Epidemiología y Microbiología. Para el diagnóstico se utilizaron los estuches de reactivos UltramicroELISA para detectar anti-HBc total, UltramicroELISA para descubrir HBsAg y su prueba confirmatoria, UltramicroELISA para revelar anticuerpos de tipo IgM al antígeno core y UltramicroELISA para detectar anticuerpos al antígeno de superficie, todos producidos por el Centro de Inmunoensayo de La Habana, Cuba.
Resultados: la mayor prevalencia se encontró en el estadio de infección resuelta, seguido del de ventana inmunológica, infección aguda e infección crónica, para un 5,25 % (n=40) de estudiantes universitarios que han tenido contacto previo con el VHB. Por otra parte, el 94,75 % (n=722) no se identificaron marcadores virales con las pruebas serológicas empleadas, definidos como susceptibles. La positividad para el estadio de infección aguda fue mayor en hombres, mientras que para el estadio de infección crónica fue mayor en mujeres.
Conclusiones: los resultados obtenidos demuestran la posibilidad de incrementar la eficiencia en el diagnóstico y la vigilancia epidemiológica del VHB.
DeCS: BIOMARCADORES; PRUEBAS SEROLÓGICAS; VIRUS DE LA HEPATITIS B; MONITOREO EPIDEMIOLÓGICO; ESTUDIANTES DE MEDICINA.
Descargas
Citas
1. Chevrier MC, St-Louis M, Perreault J, Caron B, Castilloux C, Laroche J, et al. Detection and characterization of hepatitis B virus of anti-hepatitis B core antigen-reactive blood donors in Quebec with an in-house nucleic acid testing assay. Transfusion. 2007; 47(10):1794-802.
2. Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and openlabel study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011; 55(6):1215-21.
3. Guan R, Lui HF. Treatment of hepatitis B in decompensated liver cirrhosis. Int J Hepatol. 2011; 2011:918017.
4. Bello-Corredor M, Rodríguez-Lay LA, Roodríguez-Argueta D, Montalvo-Villalba MC, Pedroso-Flaquet P, Sariego-Frómeta S, et al. Infección oculta por el virus de la hepatitis B en hijos de madres positivas al HBsAg. VacciMonitor. 2016;25(1):12-18.
5. Carneiro M, Merenho R. Natural history and clinical manifestationsof chronic hepatitis B virus. Enferm Infec Microbial Clcn. 2010;26 Suppl 7:11-18.
6. Beltrán-Durán M, Berrío-Pérez M, Bermúdez-Forero MI, Cortés-Buelvas AD, Molina-Guevara GC, Camacho-Rodríguez BA, et al. Serological profiles of hepatitis-B HBcAb-positive blood donors. 847. Rev salud pública. 2014;16 (6):847-858.
7. Reijnders JG, Rijckborst V, Sonneveld MJ, Scherbeijn SM, Boucher CA, Hansen BE, et al. Kinetics of hepatitis B surface antigen differ between treatment with peginterferon and entecavir. J Hepatol. 2011;54(3):449-54.
8. Almeida D, Tavares-Neto J, Trepo C, Almeida A, Mello C, Chemin I, et al. Occult B infection in the Brazilian northeastern region: a preliminary report. Braz J Infect Dis. 2008;12(4):310-2.
9. Allain JP, Candotti D. Diagnostic algorithm for HBV safe transfusion. Blood Transfusion.2009;7(3):174-82.
10. Norder H, Arauz-Ruiz P, Blitz L, Pujol FH, Echevarria JM, Magnius LO. The T(1858) variant predisposing to the precore stop mutation correlates with one of two major genotype F hepatitis B virus clades. J Gen Virol.2003;84(Pt 8):2083-7.
11. Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, Lankarani KB, Torab A, Moaddeb A. Anti- HBc & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res. 2006;123(1):37-42.
12. Nakamura S. Serological markers of hepatitis A and B virus infection in university students. Tokohu J Exp Med.1982;138:237-238.
13. Ramírez-Soto MC, Huichi-Atamari M, Aguilar-Ancori EG, Pezo-Ochoa JD. Seroprevalencia de hepatitis viral B en estudiantes universitarios en Abancay, Perú. Rev Peru Med Exp Salud Pública. 2011;28(3):513-7.
14. Shastry S, Bhat SS. Prevention of Post-Transfusion Hepatitis by Screening of Antibody to Hepatitis B Core Antigen in Healthy Blood Donors. Mediterr J Hematol Infect Dis. 2011;3(1):e2011062.
15. OPS/OMS. La hepatitis B y C bajo la lupa. La respuesta de salud pública en la Región de las Américas 2016. Washington, D.C.:OPS;2016.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2018 Aned Gordillo Hernández

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
La Revista Archivo Medico Camagüey, ofrece de forma inmediata después de ser indexada en el Proyecto SciELO; acceso abierto al texto completo de los artículos bajo el principio de hacer disponible y gratuita la investigación para favorecer el intercambio del conocimiento global y coadyuvar a una mayor extensión, publicación, evaluación y uso extensivo de los artículos que se exponen pudiendo ser utilizados, sin fines comerciales, siempre y cuando se haga referencia a la fuente primaria.
Carta De Declaración De Autoría u Derechos De Autor(a)
Conflictos de intereses: los autores deberán declarar de forma obligatoria la presencia o no de conflictos de intereses en relación con la investigación presentada. (Descargar Plantilla para declarar confictos de intereses)
La Revista Archivo Médico Camagüey se encuentra bajo una
Licencia Creative Commons Reconocimiento-NoComercial 4.0 International (CC BY NC 4.0).
Esta licencia permite a otros distribuir, mezclar, ajustar y construir a partir de su obra, incluso con fines comerciales, siempre que le sea reconocida la autoría de la creación original. Esta es la licencia más servicial de las ofrecidas. Recomendada para una máxima difusión y utilización de los materiales sujetos a la licencia. La licencia completa puede consultarse en: https://creativecommons.org/licenses/











