Surveillance of the effectiveness and safety of the VALERGEN vaccines in the treatment of asthma
Keywords:
inmunoterapia, reacción adversa, ácaros, asmaAbstract
Background: immunotherapy is the sole therapeutic strategy used to change the direction of physiopathology of allergic illnesses, hence the importance of this study, its application, the possible adverse reactions and its effectiveness in these conditions.
Objective: to evaluate the effectiveness and safety of the VALERGEN vaccines in the treatment of asthma.
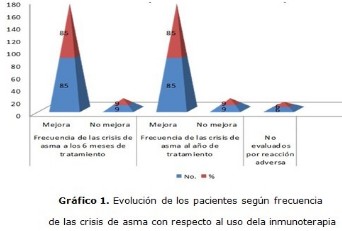
Methods: an observational study of cohort was carried out in patients with diagnosis of mild to moderate persistent and intermittent asthma on which the administration of the VALERGEN vaccines were indicated by sublingual route or subcutaneously. An active prospective approach of the patients with asthma was used and the method of pharmacosurveillance monitoring of adverse events in a cohort was established. A sample of 100 patients fulfilling the selection criteria was conformed. The data were gathered through questionnaires. The analysis of data was done using descriptive and inferential statistics. The prosecution of information was carried out by means of the statistical package SPSS version 15.0. The obtained results were illustrated in charts and graphics.
Results: a prevalence of patient with decreased asthma symptoms was found and also the need for medication with the use of immunotherapy, this result that showed a high statistical significance (p=0,000). The adverse events presented more often were, the weal >5cm, erythema, rhinitis and the rhinitis association and asthma, these prevailed in the ages from 5 to 9 years, feminine sex, subcutaneous route, with the mixture of Dermatophagoides pteronyssinus and Blomia tropicalis (VALERGEN DP + VALERGEN BT) and with concentration of 20 000 UB/ml.
Conclusions: immunotherapy with VALERGEN vaccines provides a positive clinical evolution in the majority of the patients, with a low rate of adverse events which confirms its effectiveness and safety in the treatment of asthma.
DeCS: PHARMACOVIGILANCE; DESENSITIZATION, IMMUNOLOGIC; ASTHMA/therapy; CHILD; COHORT STUDIES.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2016 Circe Pagés Rubio, Osaida Calderín Marín, María Morales Menéndez

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright: Camagüey Medical Archive Magazine, offers immediately after being indexed in the SciELO Project; Open access to the full text of the articles under the principle of making available and free the research to promote the exchange of global knowledge and contribute to a greater extension, publication, evaluation and extensive use of the articles that can be used without purpose As long as reference is made to the primary source.
Conflicts of interest: authors must declare in a mandatory manner the presence or not of conflicts of interest in relation to the investigation presented.
(Download Statement of potential conflicts of interest)
The Revista Archivo Médico de Camagüey is under a License Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 International (CC BY 4.0).
This license allows others to distribute, to mix, to adjust and to build from its work, even for commercial purposes, as long as it is recognized the authorship of the original creation. This is the most helpful license offered. Recommended for maximum dissemination and use of licensed materials. The full license can be found at: https://creativecommons.org/licenses/












 22 julio 2025
22 julio 2025