Efecto inhibitorio in vitro de fosfomicina trometamol y cefoperazona sulbactam sobre Enterobacteriaceae productoras de BLEE
Resumen
Introducción: Las infecciones del tracto urinario representan un desafío significativo en el ámbito de la salud pública, debido a la limitada disponibilidad de antimicrobianos para tratar microorganismos resistentes, como las enterobacterias productoras de β-lactamasas de espectro extendido.
Objetivo: Evaluar la actividad in vitro de fosfomicina y cefoperazona/sulbactam contra cepas de EP-BLEE aisladas de infecciones del tracto urinario y bacteriuria asintomática en el Hospital Regional Lambayeque durante el periodo comprendido entre 2018 y 2020.
Métodos: Se realizó un estudio observacional descriptivo en el que se analizaron 268 cepas de EP-BLEE, divididas en grupos según su origen, provenientes tanto de infecciones del tracto urinario como de casos de bacteriuria asintomática. La presencia de BLEE se confirmó mediante el test de Jarlier y la identificación se llevó a cabo utilizando el sistema Vitek 2. La susceptibilidad a cefoperazona/sulbactam y fosfomicina trometamol se evaluó mediante el método de Kirby Bauer.
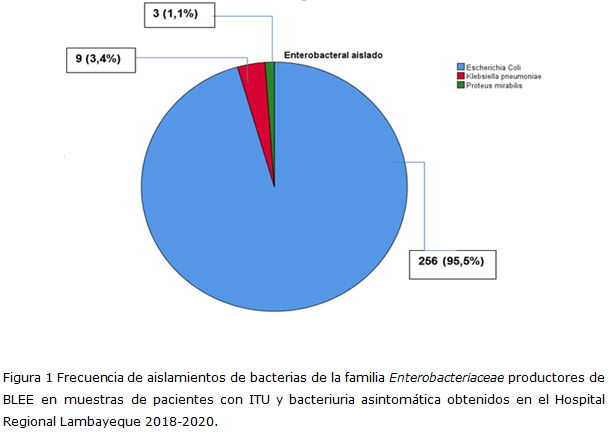
Resultados: El 95,5 % de las bacterias aisladas fueron E. coli productoras de BLEE. Según los criterios del CLSI, el 99,3 % de estas cepas fue susceptible a fosfomicina, mientras que según los criterios del EUCAST, la susceptibilidad fue del 91,8 %. La susceptibilidad a cefoperazona/sulbactam fue del 91,4 %. La media de las medidas de halos para fosfomicina y cefoperazona/sulbactam fueron 25,14 mm y 22,5 mm, respectivamente.
Conclusiones: Tanto cefoperazona/sulbactam como fosfomicina mostraron un alto efecto inhibitorio in vitro contra EP-BLEE, lo que los convierte en excelentes candidatos para estudios clínicos futuros. La susceptibilidad observada en casos de bacteriuria asintomática indica que no existe una reserva silente de resistencia a estos fármacos que pueda aumentar rápidamente con un uso más extendido.
DeCS: SUSCEPTIBILIDAD A ENFERMEDADES; INFECCIONES URINARIAS; RESISTENCIA BETALACTÁMICA; BACTERIURIA; ESCHERICHIA COLI.
Descargas
Citas
1. Tandogdu Z, Wagenlehner FME. Global epidemiology of urinary tract infections. Curr Opin Infect Dis [Internet]. 2016 [citado 9 Sep 2024];29(1):73–9. Disponible en: https://doi.org/10.1097/qco.0000000000000228
2. Pigrau C. Infección del tracto urinario [Internet]. Barcelona: SALVAT; 2013 [citado 19 Sep 2024]. Disponible en: https://www.seimc.org/contenidos/documentoscientificos/otrosdeinteres/seimc-dc2013-LibroInfecciondeltractoUrinario.pdf
3. Givler DN, Givler A. Asymptomatic Bacteriuria [Internet]. Treasure Island: StatPearls Publishing; 2022[citado 20 Sep 2024]. Disponible en: https://pubmed.ncbi.nlm.nih.gov/28722878/
4. Santamaría-Veliz O, Aguilar-Gamboa FR, Serquén-López LM, Díaz-Maldonado KC, López-Ramírez KL, Silva-Díaz H; et al. Clonalidad de cepas de Escherichia coli productoras de β-lactamasas de espectro extendido aisladas de pacientes con infección urinaria de la comunidad y portadores asintomáticos de un hospital de Chiclayo. Revista Experiencia en Medicina [Internet]. 2019[citado 20 Sep 2024];5(3):126–34. Disponible en: https://doi.org/10.37065/rem.v5i3.368
5. Aguilar-Gamboa FR. Impacto del uso irracional de antimicrobianos durante la pandemia por COVID-19. Rev Exp en Med del Hosp Reg Lambayeque [Internet]. 2022 [citado 9 Sep 2024];8(2). Disponible en: http://www.rem.hrlamb.gob.pe/index.php/REM/article/view/368
6. Maslikowska JA, Walker SAN, Elligsen M, Mittmann N, Palmay L, Daneman N; et al. Impact of infection with extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. J Hosp Infect [Internet]. 2016 [citado 9 sep 2024];92(1):33–41. Disponible en: https://www.journalofhospitalinfection.com/article/S0195-6701(15)00386-2/abstract
7. Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. Activities of Fosfomycin, Tigecycline, Colistin, and Gentamicin against Extended-Spectrum-β-Lactamase-Producing Escherichia coli in a Foreign-Body Infection Model. Antimicrob Agents Chemother [Internet]. 2013 [citado 9 Sep 2024];57(3):1421–7. Disponible en: https://doi.org/10.1128/AAC.01718-12
8. Banerjee S, Sengupta M, Sarker T. Fosfomycin susceptibility among multidrug-resistant, extended-spectrum beta-lactamase-producing, carbapenem-resistant uropathogens. Indian J Urol [Internet]. 2017 [citado 9 Sep 2024];33(2):149. Disponible en: https://doi.org/10.4103/iju.iju_285_16
9. Lai CC, Chen CC, Lu YC, Lin TP, Chuang YC, Tang HJ. Appropriate composites of cefoperazone & ndash; sulbactam against multidrug-resistant organisms. Infect Drug Resist [Internet]. 2018 [citado 9 Sep 2024]; 11:1441–5. Disponible en: https://pubmed.ncbi.nlm.nih.gov/30237728/
10. Patel B, Patel K, Shetty A, Soman R, Rodrigues C. Fosfomycin Susceptibility in Urinary Tract Enterobacteriaceae. J Assoc Physicians India [Internet]. 2017 [citado 9 Sep 2024];65(9):14–6. Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/29313570
11. World Health Organization. Antimicrobianos de importancia crítica para la medicina humana, 6.a revisión, 2018 [Internet]. Ginebra: Organización Mundial de la Salud; 2019 [citado 20 Sep 2024]. Disponible en: https://apps.who.int/iris/bitstream/handle/10665/331531/9789243515526spa.pdf?sequence=5&isAllowed=y
12. Williams PC. Potential of fosfomycin in treating multidrug‐resistant infections in children. J Paediatr Child Health [Internet]. 2020 [citado 9 Sep 2024];56(6):864–72. Disponible en: https://doi.org/10.1111/jpc.14883
13. Clinical and Laboratory Standards Institute (CLSI). CLSI M100-ED32:2022 Performance Standards for Antimicrobial Susceptibility Testing, 35nd Edition [Internet]. Estados Unidos: CLSI; 2022[citado 20 Jan 2025]. Disponible en: https://cdn.bfldr.com/YLD4EVFU/at/hvshwc8rxbsbnnmtqp9f3886/m100ed35e_sample.pdf
14. EUCAST [Internet]. The European Committee on Antimicrobial Susceptibility Testing; 2025 [actualizado 2025; citado 20 Oct 2024]. Clinical breakpoints - and guidance [about 2]. Disponible en: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf
15. Hernández Sampieri R, Fernández Collado C, Baptista Lucio MP. Metodología de la Investigación [Internet]. Mexico: Mc Graw Hill; 2014 [citado 9 Sep 2024]. Disponible en:https://www.esup.edu.pe/wpcontent/uploads/2020/12/2.%20Hernandez,%20Fernandez%20y%20Baptista-metodolog%C3%ADa%20Investigacion%20Cientifica%206ta%20ed.pdf
16. Gehringer C, Regeniter A, Rentsch K, Tschudin-Sutter S, Bassetti S, Egli A. Accuracy of urine flow cytometry and urine test strip in predicting relevant bacteriuria in different patient populations. BMC Infect Dis [Internet]. 2021 [citado 9 Sep 2024];21(1):209. Available from: https://doi.org/10.1186/s12879-021-05893-3%0A%0A
17. Sacsaquispe Contreras R, Velásquez Pomar J. Manual de procedimientos para la prueba de sensibilidad antimicrobiana por el método de disco difusión [Internet]. Lima: Instituto Nacional de salud; 2002[citado 20 Sep 2024]. Disponible en: https://antimicrobianos.ins.gob.pe/images/contenido/documentos/nacionales/manua_l_sensibilidad.pdf
18. Naushad VA, Purayil NK, Wilson GJ, Chandra P, Joseph P, Khalil Z; et al. Epidemiology of urinary tract infection in adults caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae – a case–control study from Qatar. IJID Reg [Internet]. 2022 [citado 9 Sep 2024]; 3:278–86. Disponible en: https://doi.org/10.1016/j.ijregi.2022.05.001
19. Najafi A, Hasanpour M, Askary A, Aziemzadeh M, Hashemi N. Distribution of pathogenicity island markers and virulence factors in new phylogenetic groups of uropathogenic Escherichia coli isolates. Folia Microbiol (Praha) [Internet]. 2018 [citado 9 Sep 2024];63(3):335–43. Disponible en: https://doi.org/10.1007/s12223-017-0570-3
20. Matta-Chuquisapon J, Valencia-Bazalar E, Marocho-Chahuayo L, Gonzales-Escalante E, Sevilla-Andrade CR. Presencia de genes fimH y afa en aislamientos urinarios de Escherichia coli productora de betalactamasas de espectro extendido en Lima, Perú. Rev Peru Med Exp Salud Publica [Internet]. 2020 [citado 9 Sep 2024];37(2):282–6. Disponible en: http://dx.doi.org/10.17843/rpmesp.2020.372.4829 %0
21. Stahlhut SG, Tchesnokova V, Struve C, Weissman SJ, Chattopadhyay S, Yakovenko O; et al. Comparative Structure-Function Analysis of Mannose-Specific FimH Adhesins from Klebsiella pneumoniae and Escherichia coli. J Bacteriol [Internet]. 2009 [citado 9 sep 2024];191(21):6592–601. Disponible en: https://doi.org/10.1128%2FJB.00786-09
22. Habibi M, Asadi Karam MR, Bouzari S. In silico design of fusion protein of FimH from uropathogenic Escherichia coli and MrpH from Proteus mirabilis against urinary tract infections. Adv Biomed Res [Internet]. 2015 [citado 20 Sep 2024]; 4:217. Disponible en: https://pubmed.ncbi.nlm.nih.gov/26605246/
23. Aguilar-Martínez SL, Suclupe-Campos DO, Guevara-Vásquez GM, Failoc-Rojas VE, Aguilar-Gamboa FR. Factores asociados a la colonización rectal por Enterobacteriaceae productoras de betalactamasas de espectro extendido en pacientes de consulta externa de un hospital al norte del Perú. Rev del Cuerpo Médico Hosp Nac Almanzor Aguinaga Asenjo [Internet]. 2022 [citado 9 Sep 2024];15(1):46–52. Disponible en: https://doi.org/10.35434/rcmhnaaa.2022.151.965
24. Taylor C, Abbey BS, Eszter D. What’s New from the CLSI Subcommittee on Antimicrobial Susceptibility Testing M100,29th Edition [Internet]. 2019 [ciatdo 9 Sep 2024]. Disponible en:
https://clsi.org/media/3062/clsi-update2019_21819_final_fullsizedhandouts.pdf
25. Aguilar-Gamboa FR, Aguilar Martinez SL, Cubas Alarcón DM, Coaguila Cusicanqui LÁ, Fernández Valverde DA, Mario Cecilio MM; et al. Portadores de bacterias multirresistentes de importancia clínica en áreas críticas (UCI-UCIN) de un hospital al norte del Perú. Horiz Médico [Internet]. 2016 [citado 9 Sep 2024];16(3):50–7. Disponible en: https://www.horizontemedico.usmp.edu.pe/index.php/horizontemed/article/view/470
26. Mothibi LM, Bosman NN, Nana T. Fosfomycin susceptibility of uropathogens at Charlotte Maxeke Johannesburg Academic Hospital. South African J Infect Dis [Internet]. 2020 [citado 9 Sep 2024];35(1). Disponible en: https://doi.org/10.4102%2Fsajid.v35i1.173
27. Lima RC, Faria CA, Carraro-Eduardo JC, Morales PS, Fonseca ABM. Evaluation of sensitivity profiles to fosfomycin in bacterial urine samples from outpatients. Eur J Obstet Gynecol Reprod Biol [Internet]. 2021 [citado 9 Sep 2024]]; 262:1847. Disponible en: https://pubmed.ncbi.nlm.nih.gov/34034198/
28. Hussain T, Moqadasi M, Malik S, Salman Zahid A, Nazary K, Khosa SM; et al. Uropathogens Antimicrobial Sensitivity and Resistance Pattern From Outpatients in Balochistan, Pakistan. Cureus [Internet]. 2021 [citado 17 Sep 2024]: 13(8):e17527. Disponible en: https://pubmed.ncbi.nlm.nih.gov/34646592/
29. Hussain-Gilani SY, Ali Shah SR, Ahmad N, Bibi S. Antimicrobial Resistance Patterns In Community Acquired Urinary Tract Infections. J Ayub Med Coll Abbottabad [Internet]. 2016 [citado 9 Sep 2024];28(3):572–4. Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/28712238
30. Varela EA. Rotación de antibióticos: una estrategia para paliar la resistencia. Rev CENIC Ciencias Biológicas [Internet]. 2006 [citado 9 Sep 2024];37(1):37–44. Disponible en: https://www.redalyc.org/pdf/1812/181220542007.pdf
31. Cusack TP, Ashley EA, Ling CL, Rattanavong S, Roberts T, Turner P; et al. Impact of CLSI and EUCAST breakpoint discrepancies on reporting of antimicrobial susceptibility and AMR surveillance. Clin Microbiol Infect [Internet]. 2019 [citado 9 sep 2024];25(7):910–1. Disponible en: https://doi.org/10.1016%2Fj.cmi.2019.03.007
32. Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr [Internet]. 2019 [citado 9 Sep 2024];42(1):14. Disponible en: https://doi.org/10.18773/austprescr.2019.002
33. Sardar A. Comparative Evaluation of Fosfomycin Activity with other Antimicrobial Agents against E.coli Isolates from Urinary Tract Infections. J Clin DIAGNOSTIC Res [Internet]. 2017 [citado 9 Sep 2024]; Disponible en: https://jcdr.net/articles/PDF/9440/23644_CE[Ra1]_F(RK)_PF1(PI_RK)_PFA(AK)_PF2(P_RK)_PF3(AG_OM).pdf
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Franklin Rómulo Aguilar-Gamboa, Martha Arminda Vergara-Espinoza

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
La Revista Archivo Medico Camagüey, ofrece de forma inmediata después de ser indexada en el Proyecto SciELO; acceso abierto al texto completo de los artículos bajo el principio de hacer disponible y gratuita la investigación para favorecer el intercambio del conocimiento global y coadyuvar a una mayor extensión, publicación, evaluación y uso extensivo de los artículos que se exponen pudiendo ser utilizados, sin fines comerciales, siempre y cuando se haga referencia a la fuente primaria.
Carta De Declaración De Autoría u Derechos De Autor(a)
Conflictos de intereses: los autores deberán declarar de forma obligatoria la presencia o no de conflictos de intereses en relación con la investigación presentada. (Descargar Plantilla para declarar confictos de intereses)
La Revista Archivo Médico Camagüey se encuentra bajo una
Licencia Creative Commons Reconocimiento-NoComercial 4.0 International (CC BY NC 4.0).
Esta licencia permite a otros distribuir, mezclar, ajustar y construir a partir de su obra, incluso con fines comerciales, siempre que le sea reconocida la autoría de la creación original. Esta es la licencia más servicial de las ofrecidas. Recomendada para una máxima difusión y utilización de los materiales sujetos a la licencia. La licencia completa puede consultarse en: https://creativecommons.org/licenses/











